1 Surface tension, contact angle and work Many commonly occurring phenomena that are observed in systems containing an interface in which one of the phases is a liquid can be understood through the concept of surface tension. Some examples are the rise of liquid in a narrow tube, the fact that drops of liquid tend to be spherical, and the observation that water spreads evenly on some surfaces, while remaining in isolated drops on others. In this chapter, surface tension is defined and we see how it can be used to develop simple theories to explain these experiences.
|
The bristles in a wet paint brush tend to stick together, and we might be tempted to think that the presence of water is sufficient to make the bristles stick to one another. However, if the brush is held completely under water the bristles separate (Figure 1.1), so it is not the fact that the bristles are wet, but the presence of the air–water interface that causes them to stick together.
Another example is that it is easy to make sandcastles with damp sand, but if the sand is dry or very wet it doesn’t hold together. In both cases, the stickiness depends on the presence of the air–water interface, and the phenomena can be explained by realizing that the interface acts as though it were under tension. That is, it experiences forces that pull the bristles or sand particles together. Yet another common example is that a drop of water tends to assume a spherical shape (distorted perhaps by gravity or by air resistance if falling, Figure 1.2). Now a spherical shape has the lowest surface area for a given volume of liquid, so what we observe in these and other examples is that the area of an interface tends to a minimum. The force that causes this to happen is called the surface tension or sometimes the interfacial tension. Fig. 1.2 Spherical drops falling from a round tube. If an imaginary line is drawn on a surface it will be pulled by the surfaces on either side towards those surfaces. For example, consider a partly inflated balloon (Figure 1.3). If a line is drawn on the surface, and then more air is added to the balloon, we observe that the line broadens as the rubber expands, and if the balloon were to be cut along the line the two sides would separate forming a hole.
Fig. 1.3 The tension in the rubber acts on a line in the surface, causing it to stretch.
|
Fig. 1.1 Effects of water on the fibers
|
The balloon is only an analogy, but something similar happens at the surfaces of interest to us. At any surface, if the force, F, acting tangentially to the surface and at right angles to an element, δx, of an imaginary line in the surface has a magnitude that is independent of the direction of the element, then the surface tension, γ, is:
![]() (1.1)
(1.1)
In words, the surface tension is the force per unit length acting on an imaginary line drawn in the surface. The SI units of surface tension are N /m, although because the N /m is rather large (the surface tension of the air–water interface at room temperature is about 72 mN /m), surface tension is more commonly quoted in mN /m (The C.G.S unit for surface tension is the dyn /cm, where 1 dyn/ cm = 1 mN/ m. This unit is seen in the older literature and is rarely used in current work. )
An intuitive way to understand the origin of surface tension from a molecular point of view is to consider the forces acting on a molecule at the surface of a liquid compared to those acting on one in the bulk (Figure 1.4). The attractive forces acting on one molecule in the bulk are, when averaged over time, isotropic. That is, there is no net force pulling the molecule in any given direction. A molecule at the surface, however, will feel an unbalanced force due to the relative scarcity of near neighbors in the direction of the gas phase. The result is that there is a tendency for that molecule to be pulled into the bulk, as is the case for every other molecule at the surface. Hence, the origin of the tendency to minimize the area of the surface is clear.
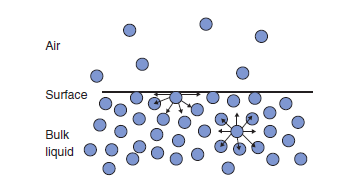
Fig. 1.4 Forces acting on molecules near a surface.
Hot keywords of USA KINO:contact angle, contact angle measurement, contact angle meter, contact angle goniometer, surface tensiometer, interfacial tensiometer, surface tension measurement, surface tension, surface tensiometry, contact angle measurement equipment and device, calculating surfac free energy, Determining Critical Micelle Concentration (CMC) of surfactant, made in China Method for choosing surface tensiometer NEW Method for choosing contact angle meter (goniometer) NEW Method for choosing interfacial tension meter NEW

Manage technical support: www.chem17.com GoogleSitemap
MainPro : contact angle,contact angle meter,contact angle goniometer,surface tension,surface tensiometer